Fei Chen, Ph.D. | March 1st, 2021
Seminar Series
Next Generation Tools for Spatial Genomics
Imaging-Based Genetic Recording of Developmental Histories
Amjad Askary, Ph.D. | April 5th, 2021
Imaging the Transcriptome: Constructing Tissues Atlases with Merfish
Jeffrey Moffitt, Ph.D. | May 3rd, 2021
Cancer Rearranges the Rules in Tissue Blocks. A New Class of Targets for Therapy?
NanoString Ultra-High-Plex Multi Omic Spatial Biology Platforms
Cancer Rearranges the Rules in Tissue Blocks. A New Class of Targets for Therapy?
10x Genomics Spatial Analysis Solutions
Spatial Epigenome Sequencing of Tissue at Cellular Level
Computational Tools for Spatially Resolved Transcriptomic Data Analysis
Past Events:
Presenter: Calum Marrs, MSci, Regional Executive – West USA at Domomite & Dolomite Bio, a Blacktrace brandTitle: Nadia IntroductionSummary: Nadia takes scRNA-Seq protocols to the next level by using automation, flexibility and an open platform to generate high quality reproducible single cell data at low cost. Nadia is an automated, microfluidic droplet-based platform for single cell research that encapsulates up to 8 samples, in parallel, in under 20 mins. Over 50,000 single cells can be captured per cartridge in a run. Nadia guides users through all relevant steps of protocols via an easy-to-use touchscreen interface, integrating sample stirring and temperature control. Adding the Nadia Innovate upgrades to the Nadia instrument into the ultimate tool for the development of novel single-cell protocols and applications.
C3-SCS (Single Cell Sequencing) Workshop Group/SocialMonday, May 6, 20194:00pm-5:00pmSanford Burnham Prebys Medical Discovery InstituteFishman AuditoriumSpeaker/Topic for Discussion are as follows:Presenter: Zhibo Ma, Ph.D., Research Associate, Wahl Group of Gene Expression Laboratory, Salk Institute for Biological StudiesTitle: Analysis of chromatin changes during mouse mammary development at single cell resolution using snATAC-seqSummary: Bipotent mammary stem cells arise during embryogenesis, and they generate the basal, luminal progenitor and mature luminal populations that found in the adult. Each adult cell population is maintained by lineage restricted progenitors after birth. Importantly, the precise time and mechanisms by which bipotent fetal mammary stem cells (fMaSC) become luminally- or basally-restricted remain to be defined. We applied single cell ATAC-seq to investigate the epigenetic reprogramming that occurs during the fetal-post natal transition, and we used an array of computational approaches to identify putative transcriptional regulators associated with the peak of fMaSC activity in mid-late embryogenesis. We generated an online resource to further enable identification of factors related to the differentiation of adult basal and luminal cells. We find that the chromatin landscape within individual cells predicts both gene accessibility and transcription factor activity. Strikingly, these single-cell chromatin profiling data reveal evidence that fMaSCs can be separated into basal-like and luminal-like lineages, suggesting early lineage segregation prior to birth. Such distinctions were not evident in prior analyses of single cell transcriptomic data. These data suggest that for mammary development, analyses of chromatin accessibility may be better a predictor of cell state potential than single cell transcriptomics.
C3-SCS (Single Cell Sequencing) Workshop Group/SocialMonday, June 3, 20194:00pm-5:00pmSanford Burnham Prebys Medical Discovery InstituteFishman AuditoriumSpeaker/Topic for Discussion are as follows:Presenter: Herbert Tseng, Ph.D., Senior Staff Researcher, Kaech Lab at Salk Institute for Biological StudiesTitle: The Single Cell Barcode Chip (SCBC), a high-throughput multiplex assay for single-cell secretome analysisSummary: Signaling through the secretion of cytokines, chemokines, and growth factors is a critical regulatory function by which cells can influence the behavior of surrounding cells and the environment around it. The functional secretome of a cell can reveal important information on cellular behavior in diseased states like tumors, and their phenotypic distribution within a tissue, such as with various immune cell subsets. That cell secretion is heterogeneous even within classically defined subpopulations, particularly polyfunctional cells that secrete multiple effector proteins, necessitates its analysis on a single cell level. Traditionally, ELISPOT assays would be used to detect the secretome, but these assays are limited in the number of proteins that can be studied, as well as throughput. Flow cytometry with intracellular cytokine staining requires fixation, and thus is not necessarily a true analysis of cell secretion. Given these limitations, the Kaech Lab has helped to develop a high-throughput multiplex single-cell secretome assay known as the Single Cell Barcode Chip (SCBC). In the SCBC, we capture single cells in PDMS nanowells that secrete onto a barcoded ELISA microarray. Using this platform, we can detect up to 15 secreted proteins of thousands of cells at a single cell level to provide a functional profile of the input cells. Dr. Tseng will discuss the development of the SCBC assay to its current form, and his progress in implementing this assay at the Salk Institute in the Kaech Lab.
Monday, July 1st, 2019 - CANCELLED
C3-SCS (Single Cell Sequencing) Workshop Group/SocialMonday, August 5, 20194:00pm-5:00pmSanford Burnham Prebys Medical Discovery InstituteFishman Auditorium (attached map, parking is free)Speaker/Topic for Discussion are as follows:Presenter: Alex Wenzel, Ph.D. Student, Mesirov Lab, University of California San DiegoTitle: Pathway expression analysis along pseudotime trajectories in single-cell RNA-seq dataSummary: Following genetic or environmental perturbations, cells may exist in different transcriptional states, reflecting functional changes in pathway activity. For a given cell type, progression through the cell cycle phases can contribute to its transcriptional states, possibly masking more subtle, cell cycle independent states. Similarly, differences in proliferation rates between populations of cells lead to overestimation of the roles of pathways coordinated with the cell cycle and undermine the identification of pathways associated with other functional differences. We developed ChromaSC, a computational method for measuring and comparing pathway activity along a pseudotime trajectory derived from scRNA-seq data. ChromaSC identifies cells engaged in the cell cycle, orders them along a pseudotime trajectory representing the cell cycle, and evaluates changes in pathway activity along this trajectory. When applied to sets of chemo-sensitive and -resistant cancer cell lines, ChromaSC allows the in silico synchronization of the cells and reveals differential pathway activities at specific times and conditions not readily discernible from current single cell differential expression methods. We will discuss future work to expand on these preliminary results, including the release of Python and R implementations of this tool.
Monday, September 2nd, 2019 - CANCELLED
Monday, October 14th, 2019
C3-SCS (Single Cell Sequencing) Workshop Group/SocialMonday, October 14th, 20194:00pm-5:00pmSanford Burnham Prebys Medical Discovery InstituteFishman Auditorium (attached map, parking is free)
Speaker/Topic for Discussion are as follows:Presenter: Carina Emery, Field Applications Scientist, Bio-Rad LaboratoriesTitle: SureCell® ATAC-Seq Library Prep Kit: A New Solution for Single-Cell ATAC-Seq using Bio-Rad’s Droplet Digital TechnologySummary: The recent advent of a single-cell Assay for Transposase-Accessible Chromatin using sequencing (scATAC-Seq) has enabled genome-wide analysis of the epigenomic landscape at single-cell resolution. scATAC-Seq can be used for a variety of applications including the unbiased discovery of new cell types and cell fate potential, and it has been suggested that mapping accessible chromatin may provide a more accurate reflection of cell type than measuring gene expression. We will present the SureCell® ATAC-Seq Library Prep Kit, Bio-Rad’s solution for scATAC-Seq. Our solution harnesses the ddSEQ Single-Cell Isolator to individually encapsulate thousands of transposed nuclei into nanoliter-sized droplets, in which barcoding and library preparation for open-chromatin sequencing occurs. The new kit employs bead overloading a novel computational strategy to utilize greater than 65% of cells in your sample with scalable cell output from 400 to 10,000 cells. The simple one day workflow uses concentrated Tn5 transposase to produce high-sensitivity, high-complexity libraries with 20,000 unique genomic fragments per cell. Come learn how your research can be a part of a new era of single-cell epigenomic studies.
2018 Workshop Group/Social Dates
Monday, March 5, 2018
Presenter: Sebastian A. Preissl, Ph.D., Associate Director, Single Cell Genomics under Dr. Bing Ren at the UCSD Center for Epigenomics in the Department of Cellular and Molecular Medicine
Title: Single nucleus analysis of chromatin accessibility in mouse for brain development
Summary: The forebrain plays a key role in multiple higher-order brain functions including memory and consciousness. Transcriptional regulatory regions in the genome including promoters and distal acting enhancers play fundamental roles for forebrain development. Regulatory elements can be identified by the presence of open chromatin as measured by ATAC-seq (Assay for transposase-accessible chromatin using sequencing). However, unraveling the role of regulatory DNA elements has proven challenging for two main reasons: 1) regulatory DNA elements are dynamic and thus their activity must be studied across multiple stages; 2) tissues are heterogeneous and comprised of different cell types with cell-type-dependent chromatin accessibility patterns. To overcome these challenges we have optimized a combinatorial barcoding assisted assay for transposase accessible chromatin for frozen brain tissue samples. Analysis of more than 15,000 forebrain nuclei at eight developmental stages reveals 20 distinct cell populations corresponding to major neuronal and non-neuronal cell types. In addition, we define cell-type-specific transcriptional regulatory sequences, infer potential master transcriptional regulators and delineate developmental changes in forebrain cellular composition.
Monday, April 2, 2018 CANCELLED
Monday, May 7, 2018 CANCELLED
Presenter: Alain Domissy, Ph.D., Scientific Director, Center for Computational Biology and Bioinformatics at The Scripps Research Institute
Title: The Metadata is your ProgramSummary: We will present a new way for experimental scientists to efficiently manage their experiments metadata and as a result gain the power to autonomously trigger and control both upstream compute intensive as well more interactive downstream analysis from the comfort of just their laptop's text editor, browser and email programs.This will be illustrated with an example of a 10x single cell rna-seq experiment. We will use a tutorial dataset which is a downsized version of a published paper, and allows going through the complete pipeline experience in less then one hour.
Monday, June 4, 2018
Presenter Raj Giraddi, Ph.D., Research Associate, Gene Expression Laboratory, Salk Institute
Title: Single cell analysis of the stem cell states
Summary: We generated a single cell transcriptome data set encompassing fetal, postnatal and adult mouse mammary epithelium and paid special attention to the perinatal interval, over which the prevalent, multipotent fMaSC phenotype declines and differentiation ensues. We elucidate biological programs that distinguish bipotent stem cells from differentiating and lineage-committed cells. Our results reveal the surprising nature of the mammary stem cell states and changes how we view the mammary gland hierarchy and its subsequent implications in breast cancer.
Monday, July 2, 2018 CANCELLED
Monday, August 6, 2018 CANCELLED
Monday, September 3, 2018 CANCELLED
Monday, October 1, 2018
Presenter: Abhishek Sohni, postdoctoral fellow in Dr. Miles Wilkinson’s laboratory, University of California, San Diego (UCSD)Title: Neonatal and Adult Human Testis Defined at the Single-Cell LevelSummary: Over 100 million men worldwide suffer from infertility. In most cases, the underlying cause is unknown. Treating human infertility requires a deep understanding of human spermatogenesis, but most of what we know about spermatogenesis comes from investigations in mice and other rodents. Given that human spermatogenesis significantly differs from rodent spermatogenesis in several aspects, we elected to perform a detailed analysis of the cell types in the human testes. Using single-cell RNA-sequencing (scRNAseq) analysis (10X Genomics platform), we identified 14 different cell types and stages, including 9 germ-cell subsets, in the adult testis. One of these germ-cell subsets appears—based on multiple lines of evidence—to be highly enriched for spermatoginial stem cells (SSCs), the only self-renewing cells in the adult testis. Several novel gene and protein markers labeling this SSC-enriched subset were identified and validated. In neonatal testes (2 and 7 days old), we identified 3 germ cell subsets, including primordial germ cell (PGC) like cells that are closely related to primordial germ cells (PGCs) from human embryos. Using pseudo-developmental trajectory analysis, we assigned cells from the PGCs stage through the PGCL and other neonatal stages to adult SSCs. We also traced the development of somatic cells from the neonatal to adult stages. Together our data delineates the identity of human testicular cells and provides a scheme of development of male germline. The SSC markers we identified hold the potential to be useful for reproductive therapies to treat infertility and testicular cancer.
Monday, November 5, 2018
Presenter: Miguel A. Tam, Ph.D., Senior Manager, Product Realization and Marketing at BioLegendTitle: Simultaneous Proteomics and Transcriptomics: TotalSeq™ and The Future of Single Cell AnalysisSummary: Welcome to the new era of single cell analysis! As personalized medicine and other highly specialized life science and medical applications continue to advance, there is an increasing demand to develop cutting edge technologies.As such, BioLegend now offers TotalSeq antibodies. These antibody-oligonucleotide conjugates seamlessly integrate with existing protocols (e.g. CITE-seq and REAP-seq) aimed at generating protein and RNA measurement simultaneously, from the same single cell. The olives provide a permanent antibody or cell tag, thanks to the barcode embedded in their sequence.Presenter: Daniel A. Peiffer, Ph.D., Executive Sales Specialist at IlluminaIllumina Updates: 1) Updates on Illumina’s newest library prep releases to enhance your research! 2) Updates on Illumina’s sequencing system portfolio including the new iSeq100!
Monday, December 3, 2018 CANCELLED
Monday, January 7, 2019
C3-SCS (Single Cell Sequencing) Workshop Group/SocialMonday, January 7, 20194:00pm-5:00pmSanford Burnham Prebys Medical Discovery InstituteFishman Auditorium (attached map, parking is free)* All are welcome and light hors d'oeuvres/drinks will be servedSpeaker/Topic for Discussion are as follows:Presenter: Dr. Sheng Zhong, Professor of Bioengineering at UCSDTitle: Rainbow-seq: Combining Cell Lineage Tracing with Single-Cell RNA Sequencing in Preimplantation EmbryosSummary: We developed the Rainbow-seq technology to trace cell division history and reveal single-cell transcriptomes. With distinct fluorescent protein genes as lineage markers, Rainbow-seq enables each single-cell RNA sequencing (RNA-seq) experiment to simultaneously decode the lineage marker genes and read single-cell transcriptomes. We triggered lineage tracking in each blastomere at the 2-cell stage, observed microscopically inequivalent contributions of the progeny to the two embryonic poles at the blastocyst stage, and analyzed every single cell at either 4- or 8-cell stage with deep paired-end sequencing of full-length transcripts. Although lineage difference was not marked unequivocally at a single-gene level, it became clear when the transcriptome was analyzed as a whole. Moreover, several groups of novel transcript isoforms with embedded repeat sequences exhibited lineage difference, suggesting a possible link between DNA demethylation and cell fate decision. Rainbow-seq bridged a critical gap between division history and single-cell RNA-seq assays.Published in iScience 2018
About the C3 Single Cell Space Force Seminar Series
Drs. Peter Adams and Geoffrey Wahl are excited to host a seminar series focused on spatial -omics and single cell technologies named “Space Force”. We plan to highlight state-of-the-art techniques for spatial transcriptomics, proteomics, and other -omic approaches to 2D or 3D mapping of normal, aged and diseased tissue. This a rapidly emerging set of technologies, transformative for most areas of biology.
C3 Single Cell Sequencing (SCS) Workshop Group / Social
First Monday of every month
5:00-6:00pm
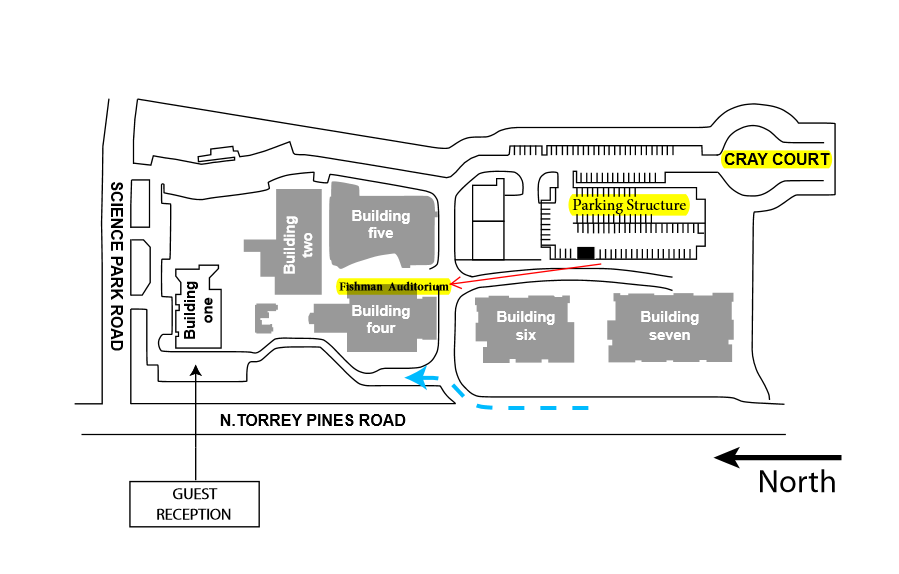
Fishman Auditorium
Sanford Burnham Prebys
10901 North Torrey Pines Road
La Jolla, CA 92037
858-646-3100
From N. Torrey Pines Road, turn on John J. Hopkins Drive; take first left into Cray Court; continue straight into the SBP parking lot. From the top level, walk north to Ruoslahti Way to Building